FDA Approves Mepolizumab As Add-on Treatment In COPD
On May 22, 2025, the United States Food and Drug Administration (FDA) approved mepolizumab (brand name: Nucala) as an add-on maintenance treatment for patients with COPD who have an eosiniphilic phenotype.
What is mepolizumab? This a biologic medication derived from living organisms or their products. They mimick cell products that occur naturally in the body. It is a monoclonal antibody that blocks interleukin-5 (IL-5) - a protein involved in inflammation in the airways. The FDA previously approved mepolizumab to treat severe asthma in patients six years or age or older.
Microscopic view of normal airway on LEFT and airway in someone with COPD on RIGHT. The pink areas in the outer parts of the airway represent inflammation. Biologic medications work in asthma and COPD by reducing airway inflammation.
How Is mepolizumab given? Mepolizumab is given by injection under the skin once a month. It can be administered in a clinic, hospital, or at home (using an autoinjector).
Why was mepolizumab approved? GlaxoSmithKline developed mepolizumab and approval was based on the results of the phase III MATINEE study. This was a randomized, placebo-controlled trial that enrolled 804 patients with COPD, a blood eosinophil count of at least 300 cells per microliter, a history of flare-ups (exacerbations), who were receiving inhaled triple therapy (two different bronchodilators and an inhaled corticosteroid). Participants received 100 mg of mepolizumab or placebo sucutaneously every 4 weeks for 52 to 104 weeks.
The results showed that mepolizumab demonstated a significant and clinically meaningful reduction of 21% in the rate of moderate or severe flare-ups compared with placebo. The time to first moderate or severe flare-up was also significantly prolonged with mepolizumab (419 days) compared with placebo (321 days) (p = 0.009). The study results were published in the May 1, 2025, issue of the New England Journal of Medicine.
What Does This Mean For Those With COPD? Mepolizumab has a place in the treatment of a subset of patients with COPD who: 1. are taking inhaled triple therapy (Breztri and Trelegy have three or triple medications); 2. have a high blood eosinohil count; and 3. at risk for future flare-ups (exacerbations) based on two flare-ups treatment as an out-patient or one or more flare-up treated in the Emergency Department or requiring hospitalization.
Certainly, you should discuss mepolizumab with your health care professional. Note that mepolizumab is the second biologic medication approved by ther FDA for add-on treatment of COPD. On September 27, 2024, dupilumab (brand name: Dupixent) was approved by the FDA for use in adults with COPD that is not adequately controlled by other medications and had an eosinophil phenotype. Dupilumab is a monoclonal antibody that blocks interleukin-4 and -13 that are involved the body’s inflammation.
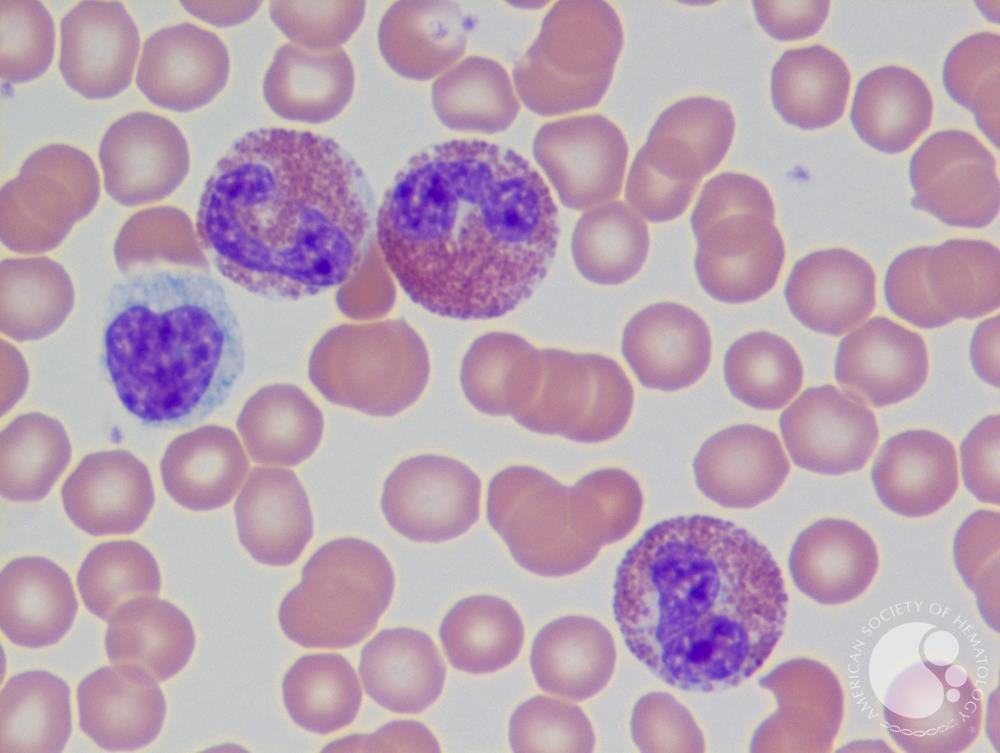
What Is An Eosinophil? It is a type of white blood cell that is part of the body’s immune system that plays a role in fighting infections, responding to allergic reactions, and involved in the body’s inflammation. It is estimated that 20 - 40% of patients with COPD have a blood eosinophil count of 300 or higher.
Microscopic view of a blood smear with three eosinophils (largest cells with purple bilobe nucleus ad pink granules within the cell). The multiple small round images are red blood cells.