Frequently Asked Questions
Dr. Mahler answers “real” questions
often asked by his patients with COPD

Clearing Airwy Mucus: Can A Flutter Valve Help?
Dear Dr. Mahler:
I am 66 years old and read your recent blog on the presence of mucus plugs in airways on CT scanning. About one month ago I had a low dose CT scan to screen for lung cancer. The nurse called and said that there was no evidence of lung cancer.
However, I signed on to the patient portal and the report stated that I had several mucus plugs in both lower lobes. I was upset that I was not told of this and had to find out myself.
My last breathing tests were nine months ago and I was told that the Trelegy inhaler was keeping my COPD stable. I am writing to ask you what should I do now to get rid of these plugs. Last winter I had two different times that my breathing got worse. On one occasion, I went to urgent care and was treated with an antibiotic and prednisone. About 6 weeks later, I got real sick, went to the ED, and was admitted to the hospital with pneumonia.
I understand from your blog that mucus plugs increase the risk for flare-ups as I have experienced. What can I do? I read about a flutter valve on the internet. Can a flutter valve help me?
Kim from Lake Placid, NY
How Many Hours Do I Need To Use My Oxygen?
Dear Dr. Mahler:
For the past 2 years, I have been using oxygen since being discharged from the hospital after a COPD flare-up. I was told to use the oxygen at a setting of 2 for 24/7. However, I find this requirement somewhat onersome.
I am 72 years old and use a nebulizer for my three different medications. I also go to a maintenance pulmonary rehab program at the local hospital here in Dayton.
My question is, How many hours do I really need to use the oxygen? When I take the oxygen off for 5 - 10 minutes, my sat stays around 87-88% and my breathing is fine. I an very conscientious about using oxygen at night when I sleep.
Thanks for your answer.
Kenny from Dayton, OH

Aging and COPD: Importance of Being Active
Dear Dr. Mahler,
I am 71 years old and understand that my COPD of four years may or may not progress based on one of your previous posts. I don’t smoke anymore, try to stay active, and am using Trelegy inhaler. This was prescribed when I was discharged from the hospital after a flare-up two years ago.
However, as I get older, I recognize both physical and mental changes that are occurring that do not appear to have anything related to my COPD. My question relates to aging with COPD - is there any information to guide or reassure me about this process. What can I expect?
Thanks,
Tomoko from Osaka, Japan
Screening For Lung Cancer: Should I Do It?
Dear Dr. Mahler:
I am 64 years old and my new physician’s assistant recommended a CT scan to screen for lung cancer. Does this make sense? I take two different inhalers in the morning for my COPD and use albuterol puffer at least once most days. Each winter I seem to get a chest infection that requires an antibtiotic and a course of prednisone. There is a family history of stomach cancer, but no lung cancer as far as I know. Thanks. By the way, I quit smoking five years ago.
Mark from Kokalakis, MI

Will My COPD Get Worse?
Dear Dr. Mahler:
I recently viewed an on-line video about COPD. One of the experts keep saying that “COPD is a progressive disease.” Is that true?
I see my lung doctor once a year and have breathing tests before each visit. Last month she showed me my test results over the past 3 years. The tests for FEV1 and FVC were quite stable over that time period. She said that I am “maintaining” my lung function probably due to the two long-acting bronchdilators that I take daily.
This is very confusing, and I am sure that others with COPD have the same question and want to know the answer.
Keep posting,
Phil from Glenview, MT

How To Boost Immune System: What Can I Do?
Dear Dr. Mahler,
I am 64 and have had COPD for about 3 years. I received two COVID vaccines this past spring, but have been reading about breakthrough infections. Even though I follow all of the recommendations to prevent exposure to the COVID-19 virus, I am worried that I will get really sick because of my COPD if I get infected. Stories on the news mention the importance of the immune system. Is there anyway that I can boost my immune system to reduce my chances of a bad outcome?
Thanks,
Sarah from Williamsburg, VA
Clearing Mucus Out Of The Chest
Dear Dr. Mahler:
I am 67 years old and have been bothered by mucus in my chest for years. I find it hard to cough it out and have tried every over-the-counter medication without any help.
A Physician Associate diagnosed me with “moderate COPD” two years ago and prescribed Advair Diskus twice a day and ProAir to use when needed. I quit smoking two years ago when I was told of COPD. In the morning, I cough out clear-gray mucus from my chest after breakfast and my 2nd cup of tea. However, there is still “rattling” in my chest afterwards which seems to last throughout the day. I try to cough out more mucus as the day goes, but it is quite difficult. I know that there is more mucus down there, but it is hard to get it out despite coughing jags.
I typically have a chest infection every winter and still work selling real estate, although I work fewer hours than I used to. Any advice is appreciated.
Victor from Topsham, UK

How To Use My Inhaler?
Dear Dr. Mahler:
It is confusing to me how to use the different inhalers prescribed for my COPD. I was diagnosed a few years ago, and my primary care started me on ProAir as needed and Spiriva HandiHaler in the morning. They helped my breathing, but I was still short of breath walking from my car to the grocery store.
I was referred to a pulmonary doctor who recommended adding Symbicort and replacing ProAir with Combivent Respimat for “rescue.” Unfortunately, the nurse who does inhaler teaching was not in the office that day. This morning, my husband watched me use the inhalers, and suggested that I wasn’t inhaling the right way. I got mad at him and told him to “Shut up.”
After I calmed down, I thought about his comment and realized that I really wasn’t sure about how to inhale correctly. It seems so simple, but I not getting the relief that I want. Please help me.
I have been quite inactive during the current COVID pandemic. I retired two years ago working as a secretary in the school district office.
Many thanks.
Karen from Carson City, NV
My COPD Inhalers Are Expensive: What Can I Do?
Dear Dr. Mahler:
I was diagnosed with COPD a few years ago and stopped working in 2020 as a cook at the local diner because it closed due to COVID. When I was working, the owner of the diner covered my health insurance and I paid $25 – $40 as co-pays for my two inhalers.
I am 66 years old and receive Social Security payments each month. I have Medicare parts A and B insurance, but part D is too expensive. My doctor has given me samples of the once daily inhaler on occasion, but he does not have albuterol samples.
I feel desperate now because I really can’t afford the two inhalers on my fixed income. Do you have any suggestions?
Ken from Sacramento, CA

More Short of Breath But Stable PFTs
Dear Dr. Mahler:
I recently saw my doctor because my breathing has been slowly getting worse. This is quite noticeable when I play golf 3 to 4 times a week. I ride in a cart and always try to get as close as possible to the tee and to my ball. My golf partners laugh about this. I also find that walking up stairs and up an incline on the golf course makes me feel short of breath.
At my recent appointment, my doctor told me that my PFTs are the same as a year ago and that my COPD is stable. He asked me about my activities since March when coronavirus caused “stay at home” orders. I told him that my husband and I have been quite inactive, and shared with him that I have gained 6 pounds since March.
My COPD medications are Stiolto in the morning and Ventolin as needed, but I rarely use this inhaler. I haven’t had a COPD flare-up for at least two years. Do you have any thoughts on why I am more short of breath?
Sincerely,
Diane from Longmeadow, MA

How Much Oxygen Should I Use?
Dear Dr. Mahler:
I am 71 years old and have severe COPD for several years, but am doing fine and have adjusted to my situation. I use oxygen 24/7 from a stationary concentrator at home and from a POC with activities and travel. I fly (at least before COVID-19) to visit my children/grandchildren in Wisconsin.
However, I am confused about my oxygen flow rate. My doctor told me that 2 liters/minute is what I need at rest, and my oxygen saturation level is usually 91-92%. However, I turn up the oxygen to 4 liters/min because I feel better when my saturation level is 94-95%.
My POC has pulse flow and I use the highest number – 5 – which maintains a saturation around 91% when I am active.
What are your thoughts on this?
Jim in Austin, TX

Why Am I Coughing So Much?
Dear Dr. Mahler:
I am 63 years old and was diagnosed with COPD about 4 years ago. For the past 3-4 months, I have been coughing more than usual. Most days I cough up white stuff, but lately there is more of it. I haven’t noticed any yellow color. It seems to come from my chest and I feel more short of breath until I cleared it out.
I told my doctor about it, but he blamed it on my smoking. I am trying to quit, but can’t cut down any more then 10 – 12 cigarettes a day. My COPD medications are Serevent Diskus twice a day and ProAir puffer 1 – 2 times a day when I feel that it is hard to breathe.
Any help is appreciated.
Bill from Avon, CT

Sexual Activity And COPD
Dear Dr. Mahler,
I am 68 years old and have had COPD for about 4 years. I am interested in having sex with my wife, but I have difficulty getting an erection. My family doctor is not too concerned as he tells me that sexual activity shouldn’t be that important at my age. He probably doesn’t understand because he is much younger.
I try to stay active most days, and quit smoking when I was told of COPD diagnosis. I don’t need oxygen.
My wife says that she is fine with or without having sex. However, she knows that I am still interested.
Do you have any suggestions?
Ray from Baton Rouge, LA
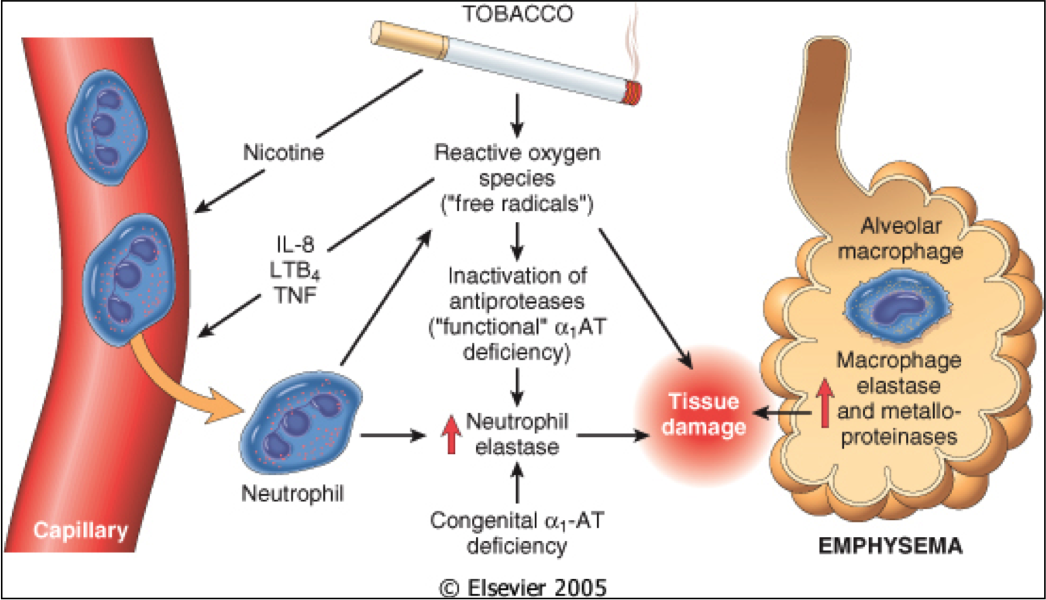
Mild Background Emphysema
Dear Dr. Mahler:
Can you tell me what is “Mild background emphysema.” Does this mean I have emphysema or something else?
Rachel from Coronado, CA

Why Short Of Breath While My Oxygen Level Stays The Same?
Dear Dr. Mahler:
I am confused – Why am I short of breath when I do various daily activities? My oxygen level doesn’t change at all when I check it. This has been going on for quite a while now.
My COPD was diagnosed 3 years ago, and I take Anoro inhaler in the morning. Previously, I was taking Bevespi inhaler, but my insurance company had me change to Anoro. The nurse at my doctor’s office has coached me on how to use the inhaler correctly. I completed a pulmonary rehab program at the local hospital, and I go to the community center 3 to 4 times a week – walking on the treadmill and pedaling on the bicycle.
What are your thoughts?
Sincerely,
Jane from Yuma, AZ

What Causes COPD Progression If I Stopped Smoking?
Dear Dr. Mahler:
I’ve read that if one stops smoking that emphysema damage doesn’t get worse. If that is correct, what causes COPD to progress in someone with emphysema who quit smoking?
Also, which has a worse prognosis, emphysema or chronic bronchitis?
Thank you for your time.
Shannon from Topeka, KS
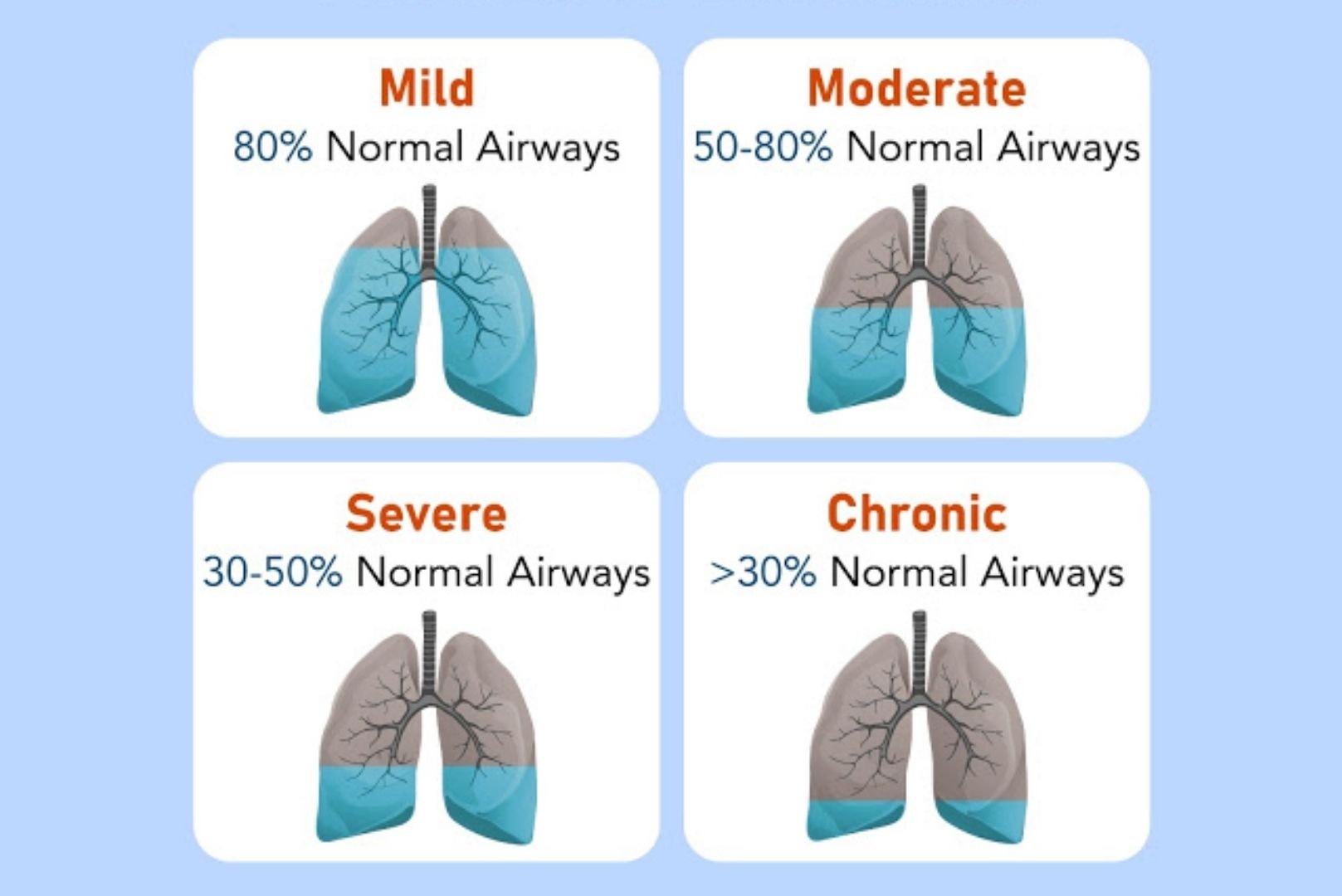
Will My COPD Progress? What Can I Expect Over The Next Few Years?
Dear Dr. Mahler:
I have read that COPD is a “progressive disease.” Does this mean that my breathing and overall condition will get worse? It would be helpful to know this in order to make plans with my family and about my finances.
Except for my “severe” COPD (according to my lung doctor), I am otherwise healthy. I take two different inhalers and go to maintenance pulmonary rehabilitation at the local hospital 2-3 times a week. Also, I try to eat healthy foods.
Thanks for any information – good or bad.
Bill from Newburgh, NY
Platelet Rich Plasma For COPD: Does It Work?
Dear Dr. Mahler,
A friend recently told me that he received a platelet rich plasma injection into his shoulder to try to repair damage to the rotator cuff muscles. He then mentioned that someone in the waiting room at the treatment center was using oxygen and told him that he was getting the same treatment for COPD. Can you tell me whether this works for COPD?
I am 71 years old and have had COPD for at least 15 years. My breathing has gotten worse and I find it difficult to even walk 30 yards before needing to stop to catch my breath. I am very frustrated because I can’t do things that I enjoy such as walking my dog on a nearby trail, and have to pay someone to do outdoor yard work on our property. I use Stiolto in the morning and ProAir several tiems a day when I feel short of breath. My oxygen level at home ranges form 91 to 93% when I do activities, and I haven’t had a flare-up in the past few years.
Should I consider platelet rich plasma treatment? I asked my lung doctor about this, but she did not know anything about this topic.
Andrew from Mt. Airy, NC

CBD Oil and Breathing
Dear Dr. Mahler:
I am writing because I want to learn more about CBD oil and whether it can help my breathing difficulty. I have had COPD for about 10 years, and find that it is getting harder to breathe. My medications are ProAir, Advair Diskus, and Spiriva powder. I used to golf 3-4 times a week, but found it too hard to play with my group this past summer. So, my main activity is playing cards in the afternoon.
One of my girlfriends was reading about CBD oil and suggested that I try it. However, I checked out information on the internet, but it is really confusing. I want to see if the oil can help me feel better and breathe easier, but I don’t want to get high.
What do you know about CBD oil for my COPD?
Many thanks.
Gloria from The Villages, FL
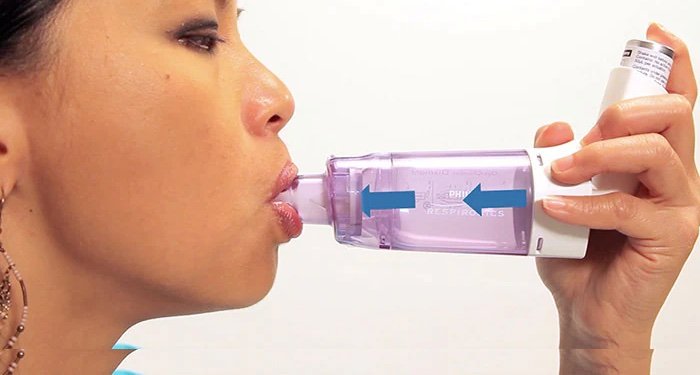
Should I Use A Spacer With My Inhaler?
Dr. Mahler:
The pharmacist at the local drug store told me that I should use a spacer with my inhalers. I am confused because Bevespi and ProAir inhalers, which I take for my COPD, seem to be working fine. I asked my nurse practioner about it, and she said that it wasn’t necessary. What are your thoughts?
Judy from Chattanooga, TN
